Enthalpies for Different Types of Reactions
Enthalpies for Different Types of Reactions: Overview
This topic covers concepts such as Enthalpy of Reaction, Standard Enthalpy of Reactions, Factors Influencing Enthalpy of Reaction, Role of Physical State of Chemicals in Enthalpy of Reaction, Enthalpy of Sublimation, Kirchhoff's Equation, etc.
Important Questions on Enthalpies for Different Types of Reactions
From the data of following bond energies:
Calculate the enthalpy of the following reaction in .
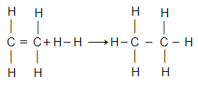
Given that bond energies of respectively and for , bond enthalpy of is:
For which one of the following equations is equal to for the product?
What is the enthalpy change for,
if heat of formation of are and respectively (in standard conditions)?
The values of heat of formation of are –298.2 kJ and –98.2 kJ. The enthalpy change of the reaction
will be
Assume each reaction is carried out in an open container. For which reaction will
The absolute enthalpy of neutralisation of the reaction:
will be:
[Consider the actual value instead of magnitude].
For the reaction:
at constant temperature, is:
How many of the following have standard heat of formation value of zero.
In Kirchhoff's equation, the term is used instead of at constant volume.
Kirchhoff's equation at constant volume is :
The variation of with temperature is expressed by Kirchoff's equation.
Temperature affects the enthalpy change of a reaction.
Write the Kirchhoff's equation at constant volume and at constant pressure.
Specific heat capacity is not the parameter that is used to relate the enthalpy of a reaction and temperature.
Describe the effect of temperature on enthalpy of reaction.
What happens to the enthalpy of the system if its temperature increases?
Give the Kirchhoff's equation at constant volume.
Which of the following is not the equation of Kirchhoff's law?
Which law relates the temperature and enthalpy change of a reaction?
